Test Method Validation (TMV) in Medical Device Manufacturing

Simon Föger
You might be wondering: "Do we really need to validate even more? Isn't this enough?"
Unfortunately, the answer is no, test methods must be validated – but for good reason.
Test method validation is not just an extra layer of confidence; it is a clear regulatory requirement.
Yes, you read that correctly – various regulations explicitly mandate test method validation in the medical device industry! This makes a lot of sense considering that all performances (acceptance criteria for products and processes) are evaluated by a test method.
What is Test Method Validation (TMV) for Medical Devices?
Test method validation (TMV) is the documented process of confirming, with objective evidence, that a test method is suitable for its specific intended use in the medical device industry.
Validation means that the method consistently produces reliable results, ensuring that regulatory requirements and quality control standards are fulfilled.
A validated test method provides confidence in the accuracy, precision, repeatability, and reproducibility of the measurement system. This understanding of measurement uncertainty is crucial, because the data generated in process validation, design verification, inspection, or monitoring activities always depend on the stability and performance of the underlying test equipment and procedures. If the measurement process itself is inconsistent, the resulting test results will inevitably vary and lose credibility.
This applies to all types of test methods: from simple attribute data inspections, where a product is compared against a reference material, to complex quantitative methods that deliver numerical measured values. Only by ensuring validated test methods can we obtain high-quality data that supports risk assessment, regulatory compliance, and ultimately patient safety.
For example, suppose a measurement system records multiple values of a defined characteristic and they cluster closely around the target value with low standard deviation. In that case, the method can be trusted to deliver reliable and repeatable data. If not, the results raise concerns about variation, suitability, and overall method performance.
In short: in the medical device field, TMV is not just beneficial, it is indispensable. Without it, process validation, design verification, and other critical activities cannot generate the level of confidence required by regulators and international organizations to demonstrate that devices consistently meet their intended purpose under stable conditions.

What is the Basic Idea of Test Method Validation (TMV)?
Imagine that the diameter of a rod is measured with a caliper. If three different people perform the measurement, you will most likely get three different results.
The reasons for these variations are manifold: the force applied to the caliper, the exact position of the measurement (since the rod is not perfectly round), the calibration and resolution of the test equipment, or other environmental and human factors. These influences create variation in the measured values, leading to uncertainty about the actual dimension.
In the medical device industry, this problem is highly relevant because reliable results and regulatory compliance are essential. Regulatory requirements such as ISO 13485 and standards issued by international organizations demand validated test methods that consistently deliver objective evidence under stable conditions and for the specific intended use of the device.
Test method validation (TMV) is the documented process to establish and demonstrate that a measurement system, including the procedure, reference standards, and test equipment, is suitable for its intended purpose.
Validation means confirmation through objective evidence that defined acceptance criteria are consistently fulfilled. This requires assessing accuracy, precision, repeatability, and reproducibility. Only under these conditions can high-quality data be generated that truly supports process validation and quality control.
Put simply, TMV ensures that a method delivers the same test results, regardless of who performs the inspection, when it is performed, or where it takes place.
Without such a validation process, the data generated cannot be considered trustworthy, and the resulting risk to patient safety and compliance would be unacceptable.
"Each manufacturer shall ensure that all inspection, measuring, and test equipment, including mechanical, automated, or electronic inspection and test equipment, is suitable for its intended purposes and is capable of producing valid results."
The keywords relevant to us are "…test equipment, is suitable for its intended purposes…" and with the definition of validation under 21 CFR Part 820.3 z) we have a requirement for test method validation [5].
"Validation means confirmation by examination and provision of objective evidence that the particular requirements for a specific intended use can be consistently fulfilled."
ISO 13485 requires under 7.4.3 [1]:
"The organization shall establish and implement the inspection or other activities necessary for ensuring that purchased product meets specified purchasing requirements."
For those arguing that it is difficult to interpret the requirement for test method validation here – how do you ensure you do not have false positive/negative results?
Other Documents like the MDSAP audit approach even state [6]:
"(...) the medical device organization may need to validate the test method used for incoming acceptance to ensure the test method is actually capable of identifying nonconforming product."
We often also hear statements like: "We follow a standardized method. We do not need to validate!"
Look into ISO 11607-2:2019, 4.4.3 [4]:
"All test methods … shall be validated … by the laboratory performing the test."
Understandably, regulations "motivate" some organizations, yet we should not forget the benefits of test method validation, as mentioned in the previous section.
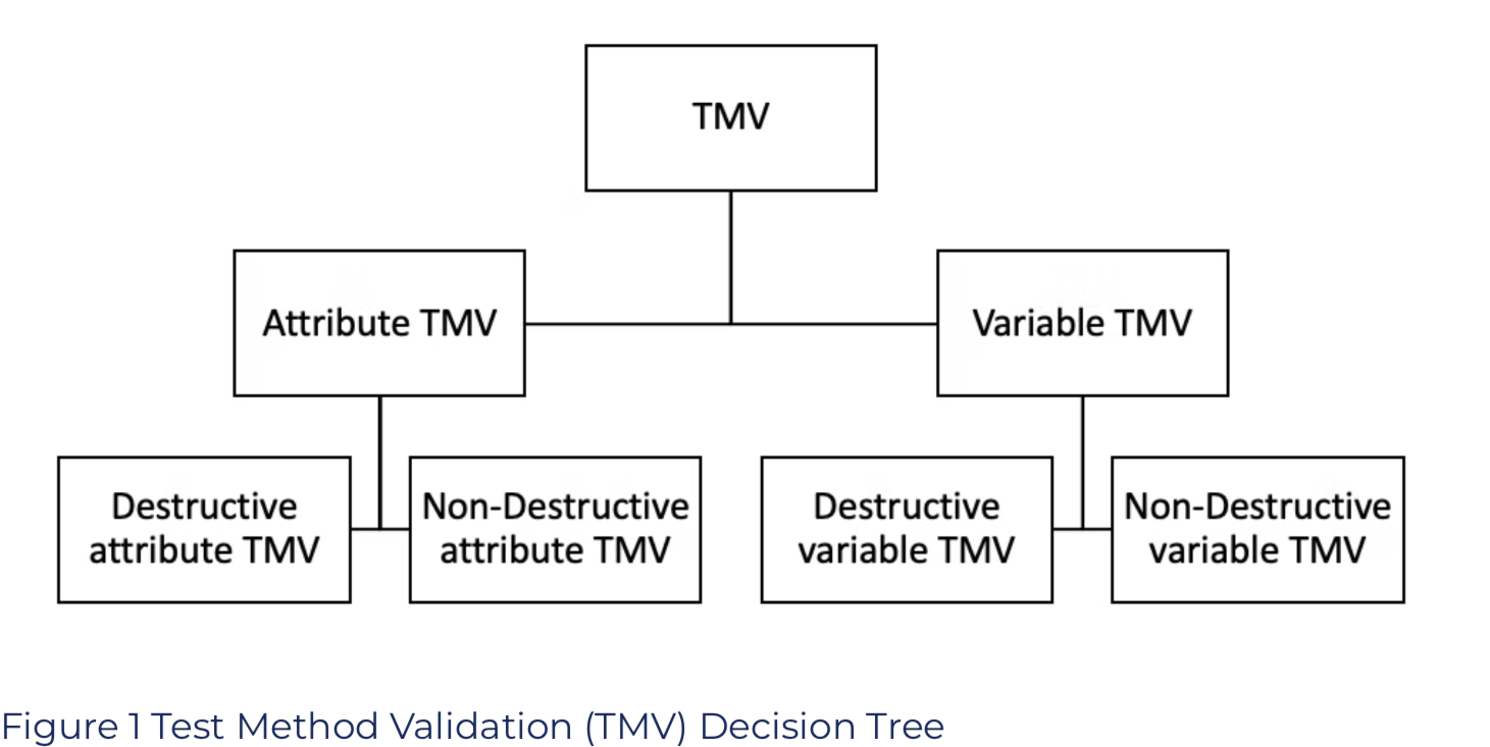
What Types of Test Method Validations Do We Have to Distinguish?
When we want to perform a test method validation, we first must distinguish what kind of data our measurement system can provide.
We have already discussed attribute and variable data in our blog post Process Validation Sample Size – Cpk of 1.33 is not enough!
Let's quickly repeat the most essential things:
- Attribute Data: go/no-go, pass/fail, good/bad; the result is qualitative.
- Variable Data: continuous values like 1.78N, 2.45mm, 1.09bar; the result is quantitative.
The following distinction we must make is whether the test is destructive or non-destructive.
Based on these two questions, we get the following decision tree:
Common Sources of Measurement Error in Medical Device Testing
You might have come across the term Gage (or Gauge) R&R (Repeatability and Reproducibility); if not, check out our blog post about Gage R&R (with Minitab).
Repeatability is the range of variation resulting from repeated measurements of the same unit by the same person, on the same day, and with the same instrument – it is the best the test method can do [2].
On the other hand, reproducibility refers to the variation that arises due to factors such as operators, days, setups, calibrations, environmental conditions, instrument conditions, and differences between instruments.
Repeatability and reproducibility together are referred to as Precision [2].
Another source of measurement error is called bias or lack of accuracy.
Accuracy refers to getting the correct values, as opposed to getting the same value.
However, bias is controlled by calibrating the instrument according to its clear requirements as specified by the U.S. FDA (21 CFR 820.72) and ISO 13485:2016 (7.6).
Summary: Test Method Validation in Medical Device Manufacturing
Key-takeaways:
- Test Method Validation (TMV) is a mandatory requirement in MedTech.
- A TMV needs to be available prior to starting OQ of process validation (or any other phase where you collect data you rely on).
- A validated test method ensures reliable and repeatable results.
This blog provides an overview of the topic of test method validation in medical device manufacturing.
If you want to dive deeper into the topic of TMV, check out our blog posts on how to perform different kinds of test method validation:

TMV Guides
Are you struggling to validate your test method? Don't let uncertainty hold you back!
Our TMV Guide streamlines the process, allowing you to validate your test methods efficiently and in compliance with MedTech regulations and standards.
Validate with confidence. Ensure reliable results. Build safer devices.

The 7 Deadly Sins of TMV
Stop reacting to audit findings – start leading.
Learn where MedTech companies repeatedly fail, what regulators truly expect, and how to set the right priorities – before inspections force your hand.
Join our free live webinar and walk away with:
- Clear insights of the 7 most common mistakes in TMV
- Practical principles you can apply immediately
- Confidence to lead TMV decisions instead of firefighting them
Get audit-ready. Gain clarity. Take the lead. Secure your seat now!
Further helpful links and resources:
SIFo Medical YouTube: Short, valuable videos on Quality Management.
Free Resources: Get free access to checklists & templates.
TMV Online Course: Become an expert in Test Method Validation.
TMV Guides: Step-by-step guides – use video tutorials and templates to validate your test methods quickly and compliant with MedTech regulations and standards.
Newsletter: Join our community and be the first to receive updates and news.
References
[1] International Organization for Standardization, ISO 13485:2016 – Medical devices – Quality management systems – Requirements for regulatory purposes, Geneva, Switzerland, 2016.
[2] W. Taylor, Statistical Procedures for the Medical Device Industry, Taylor Enterprises, Inc., 2017. [Online]. Available: https://www.variation.com
[3] Automotive Industry Action Group, Measurement System Analysis, 4th ed., 2010.
[4] International Organization for Standardization, ISO 11607-2:2019 – Packaging for terminally sterilized medical devices – Part 2: Validation requirements for forming, sealing and assembly processes, Geneva, Switzerland, 2019.
[5] U.S. Food and Drug Administration, Title 21 CFR 820.72 – Inspection, Measuring, and Test Equipment, Code of Federal Regulations, U.S. Government Publishing Office, 2023. [Online]. Available: https://www.ecfr.gov/current/title-21/chapter-I/subchapter-H/part-820
[6] U.S. Food and Drug Administration, MDSAP Audit Approach, [Online]. Available: https://www.fda.gov/media/157947/download