
This training takes you from the regulatory foundations to practical study design, data evaluation, documentation, and life-cycle management – everything you need to ensure reliable and compliant test results.

This training takes you from the regulatory foundations to practical study design, data evaluation, documentation, and life-cycle management – everything you need to ensure reliable and compliant test results.














Your Online Training in Test Method Validation (TMV) for Medical Devices
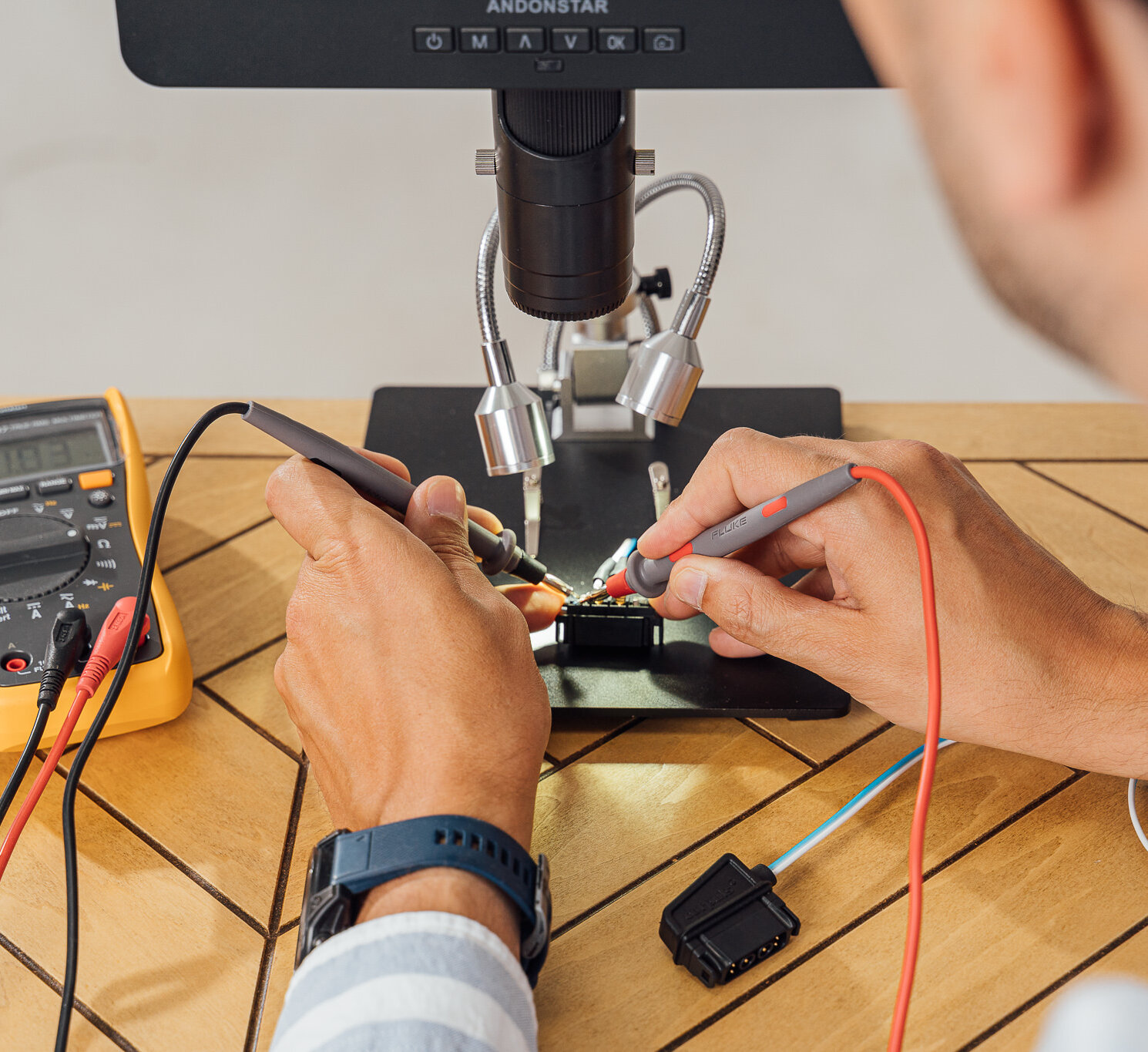
For medical device manufacturers, the journey from concept to market approval is long, complex, and heavily regulated. The one principle that governs every step is safety. A device must not only function as intended, it must do so reliably, every time.
Testing is the cornerstone of ensuring product safety. Yet here lies a widespread misunderstanding: testing alone is not enough. Many manufacturers and suppliers invest heavily in testing, but often fail to validate their test methods. Without validation, even the most sophisticated tests can generate misleading results.
The consequence? Faulty products in the field, batch rejections, wasted resources, and in the worst cases: costly recalls and reputational damage.
This is why training in Test Method Validation (TMV) is so crucial. It provides the knowledge and framework to select the right test methods, validate them systematically, and demonstrate compliance to regulators, notified bodies, and customers.

Simon Foeger is a recognized expert in medical device quality and validation, trusted by leading organizations across Europe. As a trainer at TÜV SÜD Academy, he has educated countless professionals in Test Method Validation (TMV), process validation, and statistics in MedTech.
With years of hands-on experience in the medical device industry, Simon combines deep regulatory knowledge with a pragmatic approach. He knows the realities manufacturers face (tight timelines, strict auditors, and complex requirements) and translates them into clear, practical solutions.
What makes Simon stand out as a trainer is his ability to make complex topics simple. Participants consistently value his structured explanations and relatable examples. Instead of overwhelming theory, Simon ensures that every concept is connected to the daily challenges of medical device development and compliance.
Whether you are new to TMV or seeking to sharpen your expertise, Simon provides the insights and confidence you need to validate test methods effectively, and to demonstrate compliance with regulators, notified bodies, and customers.
Your Roadmap to Become an Expert in Test Method Validation
The Test Method Validation (TMV) Training is divided into six structured modules, each building on the previous one. For every module, you'll get detailed presentation slides and a video tutorial – giving you the flexibility to learn at your own pace and revisit key topics anytime to deepen your understanding.
What Clients Say About Working With Us
Who This TMV Training Is For
Frequently
Asked
Questions
This training is designed for MedTech professionals (e.g. QA/RA managers, validation engineers, product developers, supplier quality engineers) and suppliers working with or within medical device companies.
It's also ideal for professionals from related industries (like automotive or pharma) looking to transition into MedTech.
- A video tutorial explaining key concepts and examples
- Downloadable presentation slides