Our Test Method Validation (TMV) Guides provide a clear and structured approach to ensure your test methods for sterile medical packaging are accurate, repeatable, and compliant.

Our Test Method Validation (TMV) Guides provide a clear and structured approach to ensure your test methods for sterile medical packaging are accurate, repeatable, and compliant.














Test Method Validation Guides for
Sterile Medical Packaging

When packaging processes or test methods fail, the consequences can be severe, ranging from product contamination to risks for patients.
As a medical device manufacturer, you must ensure that your packaging is safe and reliable to maintain the sterility of your products.
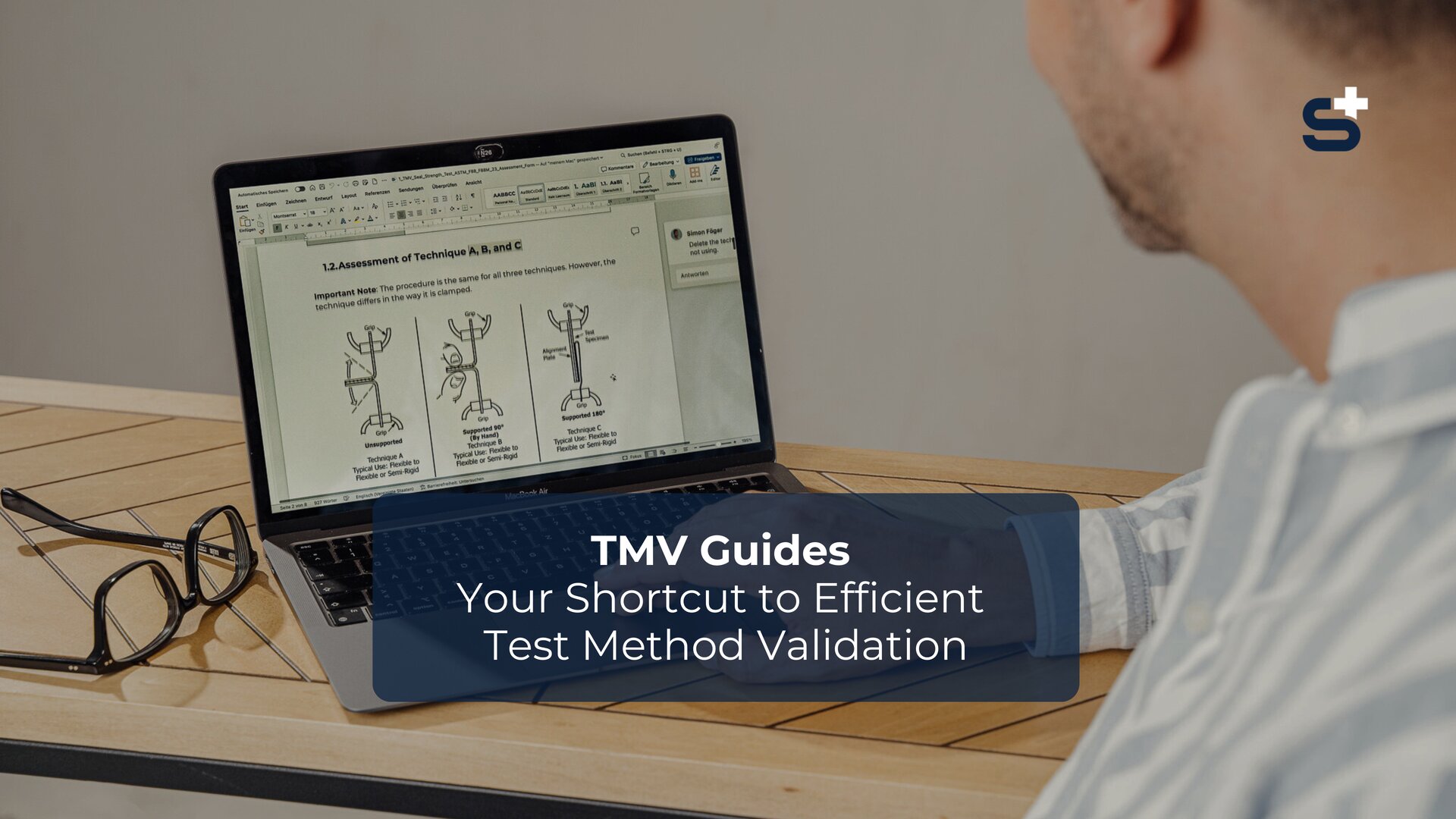
To meet regulatory requirements and increase patient safety, it is essential not only to perform test methods such as dye penetration tests, seal strength tests, bubble leak tests, or visual inspections but also to validate them thoroughly.
Our TMV Guides provide you with step-by-step video tutorials and ready-to-use templates to carry out these validations efficiently and in full compliance with the standards.
Trusted by Medical Device Professionals
Read how our clients accelerated their validation success with our expert support in test method validation.
MedTech Blog
Explore our detailed blog posts covering key topics such as the importance of TMV, how to develop a test method, how to run a Gage R&R study in Minitab, and much more.
Frequently
Asked
Questions
With our Test Method Validation Step-by-Step Guide, the estimated workload is around 8 hours, i.e. one working day. In addition, there is the sample preparation. Of course, the time required also depends largely on your internal processes (approval cycles, etc.).
The validation of test methods is a process in which we check whether a test method provides reliable results and is suitable for use in medical technology.
Correctly implementing a test method validation reduces the risk of faulty products on the market.
Test method validation is not just a requirement; it's crucial if you want to manufacture safe medical devices.
The most important medical technology standards, such as ISO 11607, ISO 11608, or MDSAP, mandate the validation of your test method, underlining the gravity of this process.
For two main reasons:
1) Test Method Validation is mandatory in the medical device industry.
All essential standards require a TMV (e.g., ISO 13485, ISO 11607, ...). In the MDR 2017/745 there is no direct requirement for a TMV, but indirectly (e.g.) through the reference in Article 10.9: "Manufacturers must have an appropriate quality management system (QMS)".
2) Validation of your test method massively increases the safety of your medical devices.
By validating your test method, you ensure its reliability. Regardless of who tests the devices, when, or where, you will reliably get the same result.
Certain standards must be followed when performing a test method, e.g., ASTM F1929 if you are performing a dye penetrant test or ASTM F88/F88M if you want to check the seal strength.
Yes, essential regulations and standards require test method validation. You should validate your test methods not only because standards require it but, above all, because it is a necessary process that contributes to the safety of your medical devices
Navigating the maze of directives and standards to understand what's truly required can be overwhelming. When it comes to implementation, uncertainty often follows, raising critical questions with no clear answers.
Some turn to external consultants. Others attempt to validate test methods internally, unsure whether their approach will satisfy regulatory expectations.
But when it comes to medical device safety, guesswork is not an option.
The TMV Guide provides a clear, structured path for conducting test method validation correctly and in full compliance with applicable standards and regulations.
Designed for professionals with or without prior experience, this guide includes ready-to-use protocols and the essential knowledge needed to carry out a TMV confidently, efficiently, and audit-ready.
You will receive all necessary templates and video tutorials to validate your test methods acc. to available standards.
We explain step-by-step, using video instructions, how to validate your test method and how to fill in the pre-made templates correctly.
What you'll get:
The TMV step-by-step guide is designed so that you can carry out the test method validation without any prior knowledge, within one working day (excluding time for sample preparation).
Optional:
On request (to office@sifo-medical.com) you can order a starter kit for carrying out the dye penetration test (ASTM F1929) from us.
We will provide you with the necessary utensils:
At SIFo Medical, we developed the Online Step-by-Step Guide to Test Method Validation in response to a clear need from MedTech manufacturers: a practical, structured approach for implementing TMV.
Many teams told us they were missing a clear, actionable framework to confidently carry out test method validation without uncertainty or over-reliance on external consultants. MDR 2017/745 and related standards place high demands on manufacturers and the workload can be overwhelming.
This guide offers a ready-to-implement solution to help you validate your test methods in a way that is quick to implement, compliant and efficient.
The TMV Step-by-Step Guide is suitable for quality managers and employees in quality management at medical device manufacturers.
Whether experienced in MedTech or new to test method validation, the TMV Step-by-Step Guide is doable for everyone.
The TMV online guide is designed so you can carry out your test method validation in practice quickly and comply with all legal regulations and standards.
This is not a theory course but rather a practical, step-by-step guide that you can follow without any prior knowledge.
If you are expecting a theory course, then this course is not suitable.
We currently offer the test method validation step-by-step guide for the following test methods:
Add the desired guides to your cart and enter your details. You will then receive an offer by email, which you can review and approve internally if needed.
Once the offer is confirmed, you’ll receive the invoice. As soon as the payment is received, access to the TMV Guide will be activated, and you can get started right away.
If you have any questions during the ordering process, please contact Sophia at office@sifo-medical.com.
The dye penetration test, according to ASTM F1929, is a standard test method for checking whether the seal seam of your packaging is tight.
To check this, you put a special test ink in the packaging and visually check whether the ink penetrates through the seal seam.
According to ASTM F1929, you can choose between three different methods of performing this test (injection method, edge dip method, or eyedropper method).
Further information about the standard can be found at: https://www.astm.org/f1929-15.html
According to ASTM F2096, bubble leak testing is a standard test method for detecting significant leaks in packaging.
It is a destructive test method that is suitable for tray and bag packaging.
Further information about the standard can be found at: https://www.astm.org/f2096-11r19.html
This standard test method checks the seal seam strength of the packaging.
This measures the force necessary to separate a test strip from the seal material. The test can be carried out on sealed seams between flexible and rigid materials.
Further information about the standard can be found at:
During the visual inspection, you check the sealing seams of your packaging.
Pay particular attention to areas that have not been sealed properly, such as wrinkles, holes, or tears in the sealing seam.
Further information about the standard can be found at: https://www.astm.org/f1886_f1886m-16.html
You can download our general terms and conditions for digital content here:General Terms and Conditions