TMV Training




Gain full confidence in your TMV process – develop audit-ready, compliant test methods that meet EU and FDA expectations.
Online Training in Test Method Validation (TMV) for Medical Devices
Training contents
2 hrs. 25 min.
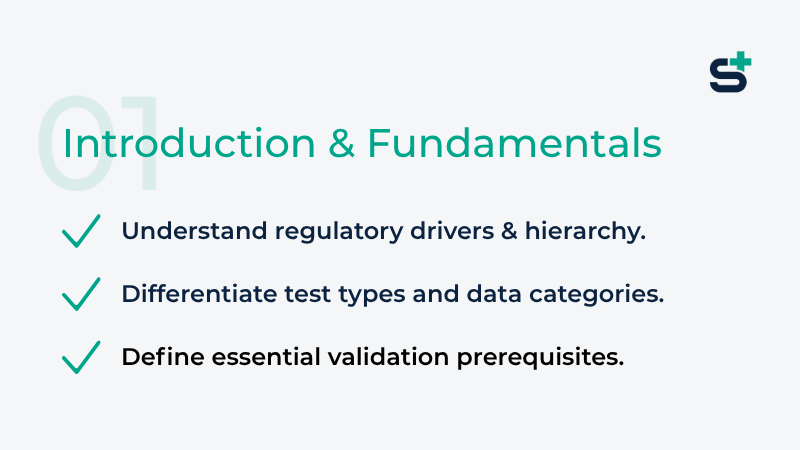
Module 1: Introduction & Fundamentals

Buy this item to view the content
The foundation of every compliant validation: In this module, you'll uncover the regulatory framework that demands TMV, learn to classify test types and data, and define the prerequisites that make or break your validation. Mastering these basics means fewer surprises later and validations that pass on the first try.
Downloads:
Module_1_Introduction_Fundamentals_TMV_Training_Slides.pdf
3.96 MB
PDF
25 min.
Module 2: Planning the Validation

Buy this item to view the content
Avoid the gaps auditors love to find: Revalidation forgotten?Acceptance criteria unclear? Missing rationale for parameters? Module 2 shows you exactly how to prevent these common (and critical) findings.
You’ll learn when validation or revalidation is required, how to create a solid TMV plan, and how to document parameters and acceptance limits so that no auditor can question your logic. A solid plan today prevents major findings tomorrow.
Downloads:
Module_2_Planning_the_Validation_TMV_Training_Slides.pdf
5.62 MB
PDF
30 min.
Module 3: The Study in Detail

Buy this item to view the content
Master Test Method Validation with confidence: This module shows you how to prove, with data, that your method is accurate, precise, and reliable. You'll master the difference between accuracy, precision, trueness, and bias, design robust studies for variable and attribute data, and interpret Gauge R&R results with confidence.
Downloads:
Module_3_The_Study_in_Detail_TMV_Training_Slides.pdf
17.54 MB
PDF
30 min.
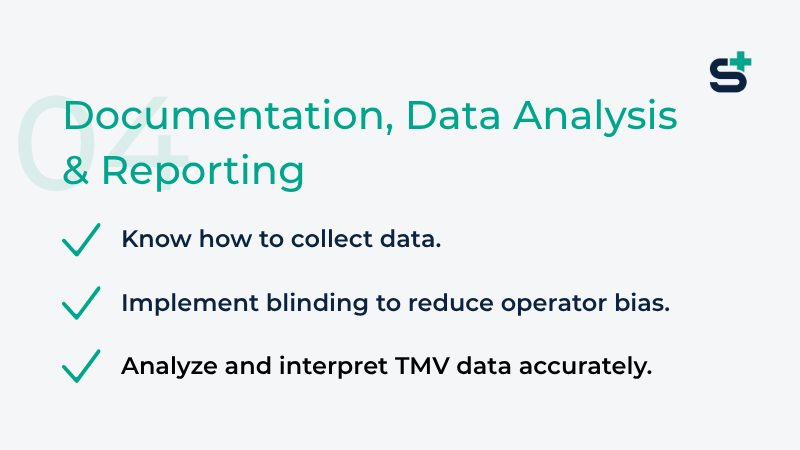
Module 4: Documentation, Data Analysis & Reporting

Buy this item to view the content
If it is not documented, it does not exist: learn how to collect data systematically, apply blinding to minimize operator bias, and analyze and interpret results with accuracy and confidence.
Downloads:
Module_4_Data_Analysis_Documentation_Report_TMV_Training_Slides.pdf
6.23 MB
PDF
25 min.
Module 5: Life Cycle Management

Buy this item to view the content
Keep your test methods fit for intended use: master the principles of test method lifecycle management – from monitoring ongoing performance to managing changes with a risk-based mindset. Learn how to take ownership of lifecycle control and move from reactive firefighting to proactive quality leadership.
Downloads:
Module_5_Life_Cycle_Management_TMV_Training_Slides.pdf
1.71 MB
PDF
15 min.
Module 6: Summary & Practical Tips

Buy this item to view the content
From knowledge to practice: understand the big picture and integrate everything you've learned. Learn from practical examples and define a path for continuous improvement across your organization.
Downloads:
Module_6_Summary_Practical_Tips_TMV_Training_Slides.pdf
2.72 MB
PDF
20 min.
Who is this training suitable for?
This TMV Training is ideal for MedTech professionals – including QA/RA Managers, Validation Engineers, Quality Engineers, Process Engineers, and Supplier Quality Engineers – who are in charge of validating test methods. The training is also suitable for suppliers to MedTech manufacturers and professionals from related industries, looking to change careers into MedTech.
Prerequisite
No prior knowledge is required but a general understanding of the regulatory framework is recommended.