Medical Device Process Validation Explained: IQ, OQ, PQ with Practical Examples & Compliance Tips

Simon Föger
Has your notified body or customer suddenly asked for process validation documents, and you're scrambling to respond? You're not alone. Many MedTech manufacturers face this exact challenge, often too late in the game.
Process validation remains one of the top FDA inspection findings, with 21 CFR 820.75 frequently landing among the top 3–5 deficiencies in warning letters.
That's no coincidence – it's a clear indicator that this critical requirement is still misunderstood or neglected.
In this article, we provide a concise yet relevant guide to process validation in medical device manufacturing. We will walk you through the fundamentals – from the definition of process validation to the regulatory requirements, what IQ, OQ, and PQ mean, and what the validation master is all about.
While the GHTF (Global Harmonization Task Force; IMDRF - International Medical Device Regulators Forum) published excellent guidance on process validation, very few seem to know or follow it.
But let's start from the beginning ...
What is Process Validation?
The U.S. FDA defines Process Validation as "establishing by objective evidence that a process consistently produces a result or product meeting its predetermined specifications."
To achieve this, organizations must establish protocols and acceptance criteria to ensure the process consistently produces results that meet all required specifications.
"Process Validation means establishing by objective evidence that a process consistently produces a result or product meeting its predetermined specifications."
Why Do Notified Bodies Require Process Validation for Medical Devices?
Process Validation isn't a new requirement introduced by the European MDR (Medical Device Regulation). The requirement for Process Validation has been around for many years, in fact, multiple decades!
Standards like ISO 13485:2016 (Section 7.5.6) and regulations, such as the U.S. FDA's 21 CFR 820.75, explicitly require manufacturers to validate processes, especially when outputs cannot be fully verified through subsequent inspection or testing.
However, the regulatory requirements should not be the only reason why you should be validating your processes.
Process validation indicates:
"that a process has been subject to such scrutiny that the result of a process (a product, service, or other outcome) can be practically guaranteed" [1].
Process validation involves intense scrutiny of manufacturing processes to guarantee consistent product quality.
This is crucial, especially when process-related defects only become evident when the product is used. So it's in your best interest to know your processes.
In short, understanding and validating your processes isn't just about satisfying auditors – it's about protecting patients, reducing risk, and avoiding costly surprises down the line.
"Process Validation indicates that a process has been subject to such scrutiny that the result of a process (a product, a service or other outcome) can be practically guaranteed [1]."
"The organization shall validate any processes for production and service provision where the resulting output cannot be or is not verified by subsequent monitoring or measurement and, as a consequence, deficiencies become apparent only after the product is in use or the service has been delivered."
Well, this should answer our question.
An organization must validate processes if they do not fully verify its output.
This may explain why some in the industry believe that only those processes requiring destructive testing must be validated.
While it's true that processes involving destructive testing often require validation, destructive testing itself is not the deciding factor.
Take, for example, seal strength testing of sterile barrier systems. If you destructively test each unit to verify the seal, the pouch cannot be reused after the test and you'll be left with little to sell. In such cases, validation is the only way to ensure product integrity without testing every item.
On the other hand, just because a product characteristic can be verified non-destructively doesn't mean validation isn't still required.
The question you should be asking is:
Do I verify a process output or product characteristic to 100%?
- If yes, validation is not required.
- If no, you must validate your process – always.
Attention: This principle applies broadly, but certain processes are explicitly expected to be validated regardless.
The U.S. FDA, for example, considers sterilization, aseptic processing, injection molding, and welding as processes that require validation [3].
The GHTF adds clean room environmental conditions, sterile packaging sealing processes, lyophilization, heat treating, and plating to the list of processes which should be validated [1].
Chances are, the outputs of these processes cannot be fully verified, which is precisely why validation is necessary.
In summary, determining whether validation is required starts with a single question: Is the output of the process fully verified? If not, you must validate.
IQ, OQ, PQ – The Three Pillars of Medical Device Process Validation
Now we're getting into the heart of medical device process validation: IQ, OQ, PQ.
These three terms are often the first things people know when process validation comes up – and rightly so. The three core stages of medical device process validation are:
Installation Qualification (IQ) is performed for equipment and machines to ensure reliable equipment operation [3]. The manufacturer is responsible for ensuring that equipment is installed and validated according to specifications to prevent non-conformities and delays.
While the Operational Qualification (OQ) ensures that all predetermined product requirements are met at process limits, it also uses activity triggers to verify dynamic process attributes, automation features, and safety checks, confirming that processes run correctly within specified operating conditions.
The Performance Qualification (PQ) ensures that all predetermined product requirements are met consistently, and PQ tests the process under normal (or anticipated) operating conditions to ensure consistent results. PQ also verifies that the final product meets all specified requirements.
This indicates that multiple processes can run on the same equipment. As such, it's essential to distinguish between "machine" and "process".
In practice, these terms are frequently used interchangeably, which leads to confusion and makes process validation challenging to understand.
The following figure illustrates the point at which the actual manufacturing process commences.
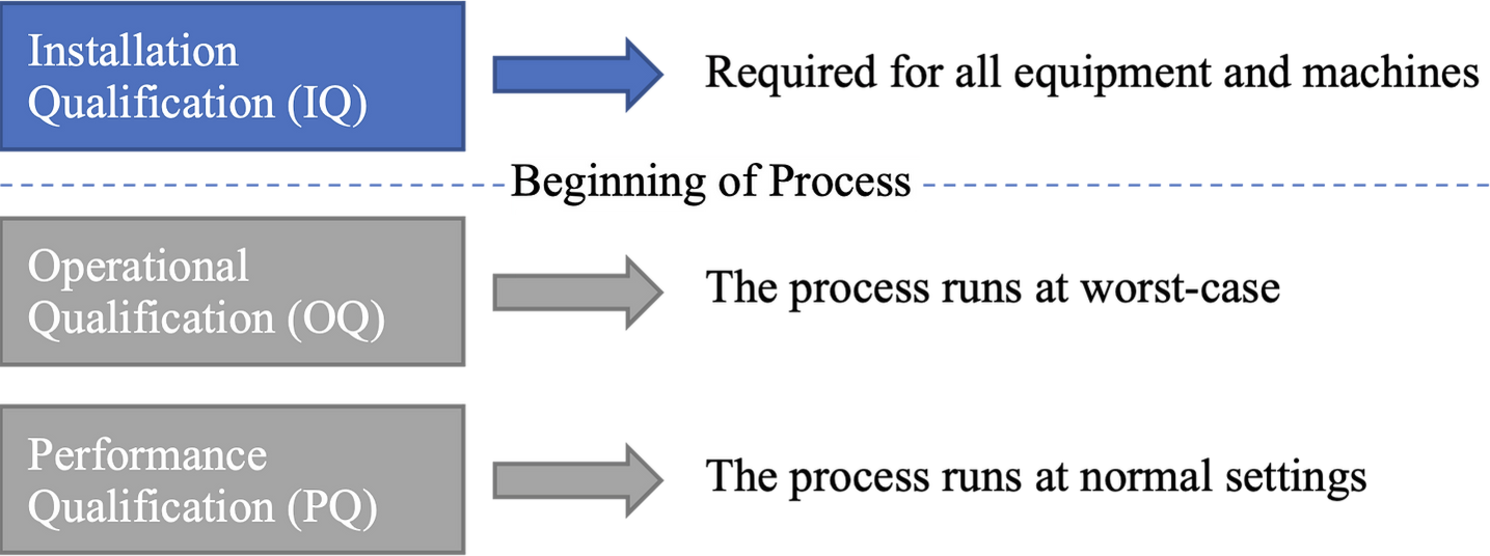
Each phase – IQ, OQ, and PQ – is substantial enough to deserve its own deep dive.
For a more detailed look at each stage of the medical device process validation, explore our dedicated articles on:
The Validation Master Plan: Your Roadmap for Medical Device Process Validation
The Validation Master Plan is another commonly used term that is often misunderstood.
The Validation Master Plan (VMP) – sometimes referred to as a Master Validation Plan – is a tool for planning and monitoring all validation activities. A VMP often takes the form of an official document or template used to record validation activities, ensuring all necessary steps are documented and compliant.
While no regulation explicitly mandates the creation of a VMP it is strongly recommended by the GHTF [4] and widely recognized as a best practice in medical device process validation.
A well-structured VMP typically includes listings of equipment and processes to be qualified or validated, regardless of whether the process is performed in-house or outsourced. The VMP also provides a framework for scheduling, responsibility assignment, and documentation control.
Beyond its planning function, the VMPs also serve to demonstrate management's commitment to validation activities and thus support the planning and allocation of human and financial resources, which are indeed required by regulations [3].
And as we've only scratched the surface of the acronyms, it becomes clear:
Medical Device Process Validation is much more than just IQ, OQ, and PQ.
Benefits of Process Validation in Medical Device Manufacturing
Process validation is more than just a regulatory checkbox – it's a cornerstone of success in the medical device industry. By thoroughly verifying that your manufacturing processes are operating correctly and consistently, you not only meet FDA requirements and ISO 13485 standards but also establish a foundation for long-term quality and compliance.
One of the most significant benefits of process validation is the reduction of risk. When you validate your processes, you proactively identify and address potential issues before they can impact device quality or patient safety. This means fewer product recalls, less rework, and a stronger reputation for your company. In a field where trust is everything, demonstrating a robust validation process can set you apart from competitors.
Process validation also helps manufacturers save time and resources. By ensuring that your manufacturing process can produce devices that meet predetermined specifications, you minimize the likelihood of costly production delays or failures. This efficiency translates into lower production costs and less waste, allowing you to focus on innovation and growth.
Moreover, a well-documented validation process is a clear signal to regulators, customers, and partners that your company is committed to delivering safe, high-quality medical devices. It shows that you take compliance seriously and are dedicated to producing devices that perform as intended, every time.
In short, process validation is not just about meeting regulatory requirements, it's about building a culture of quality that benefits your business, your customers, and ultimately, the patients who rely on your products.
Challenges in Process Validation
While the advantages of process validation are clear, implementing an effective validation process can be challenging for many manufacturers in the medical device industry.
One of the primary hurdles is determining when a manufacturing process cannot be fully verified through subsequent inspection or testing. This requires a deep understanding of both the process itself and the specific design specifications of the medical device.
The validation process often involves extensive documentation, rigorous testing, and careful analysis, all of which can be time-consuming and resource-intensive. For new processes or equipment, developing robust validation protocols that satisfy regulatory requirements can be particularly demanding. Even with existing processes, any significant change, such as a modification to equipment, materials, or software, can trigger the need for revalidation, adding another layer of complexity.
Software used in medical device production presents its own set of challenges. Validating software requires specialized expertise to ensure that it functions reliably within the manufacturing process and does not introduce unforeseen risks. Keeping up with evolving regulatory requirements and standards further complicates the process, as manufacturers must continuously adapt their validation strategies to remain compliant.
Ultimately, overcoming these challenges requires a thorough understanding of the validation process, a commitment to meticulous documentation, and the ability to anticipate and respond to changes in both technology and regulations. By addressing these obstacles head-on, manufacturers can ensure that their processes remain robust, reliable, and fully compliant.
Best Practices for Effective Process Validation
To maximize the effectiveness of your process validation efforts, it's essential to follow best practices that prioritize quality, safety, and regulatory compliance from the outset.
Start by gaining a comprehensive understanding of your manufacturing process and the product's documented design specifications. This includes mapping out the initial design control process and the entire process development sequence, so you know exactly what needs to be validated and why.
Developing a validation master plan is a critical step. This plan should clearly define the scope, objectives, and acceptance criteria for each validation activity, ensuring that all parties involved understand what success looks like. Incorporate risk assessments to identify critical process variables and potential failure points, and use objective evidence, such as data from testing and monitoring, to verify that your process is operating correctly and producing consistent results.
Tip: You can download a ready-to-use Validation Master Plan template in our Free Resources section.
It's also important to ensure that all equipment is installed correctly and functioning as intended before moving forward with validation. Regularly monitor and maintain your manufacturing processes to confirm they remain within the established operating range and continue to meet user needs and intended uses. Document every step of the validation process thoroughly, as this not only supports compliance with regulatory requirements but also provides a valuable reference for future process improvements or audits.
By establishing proper controls, maintaining clear documentation, and continuously verifying process performance, manufacturers can produce high-quality medical devices that meet both regulatory standards and customer expectations.
Effective process validation is not a one-time event – it’s an ongoing commitment to excellence in every device you manufacture.
Further helpful links and resources:
SIFo Medical YouTube: Short, valuable videos on Quality Management
Free Resources: Get free access to checklists & templates (like a validation master plan)
TMV Guide: Your practical guide to perform test method validation (incl. templates & videos)
Newsletter: Join our community and be the first to receive updates and news.
Do you have any questions about medical device process validation? Contact us at office@sifo-medical.com – we'll gladly support you on your way to compliant processes.
References
[1] GHTF/SG3/N99‑10:2004, Quality Management Systems – Process Validation Guidance (January 2004), S. 2–3. URL: http://www.imdrf.org/docs/ghtf/final/sg3/technical-docs/ghtf-sg3-n99-10-2004-qms-process-guidance-04010.pdf
[2] ISO 13485:2016 – Medical devices — Quality management systems — Requirements for regulatory purposes. International Organization for Standardization, Geneva, 2016.
[3] https://www.fda.gov/medical-devices/quality-system-qs-regulationmedical-device-good-manufacturing-practices/medical-devices-current-good-manufacturing-practice-cgmp-final-rule-quality-system-regulation
[3] http://www.quality-on-site.com/get.php?fileid=139
[4] https://www.fda.gov/media/94074/download
